and the
Both fail to explain the overwhelming 2 abundance because the free electron densities are low in dense clouds.
Dust plays a tremendous role in interstellar chemistry on all levels, particularly in dense molecular clouds. Many of the physical properties of dust and the effect these properties have on the processes are poorly understood. This paper examines some recent theories on how grains adsorb gas-phase species, process molecules and atoms, and re-release them back into the gas phase. Though models have been created, the number of free parameters present leads to a wide variety of conclusions. In this author's view, more fundamental research is needed into the properties of interstellar grains before truly predictive models can be produced.
Current theory and observations show grains forming in the outflows of giant stars. Even at this early stage they act to effect later chemistry by locking up elements. Estimates have around 20% of the available oxygen, 50% of the carbon and practically all of the silicon and iron being bound up into dense, high-evaporation-point cores which thence become chemically inert (Williams 87).
One of the most widely recognized functions of grains is the shielding of dense molecular clouds from UV radiation field. This screening effect allows the temperatures with these clouds to become quite low (10-30K) and limits the ionization of species within. This dense, cool cloud in turn can collapse to form new stars.
Another is the production of 2. The canonical evidence for the existence of grains is the overwhelming abundance of 2. Molecular hydrogen can be formed in the gas phase by the ![]() route,
route, ![]()
and the ![]() route,
route,
![]()
Both fail to explain the overwhelming 2 abundance because the free electron densities are low in dense clouds.
The outstanding favorite for 2 formation is grain catalysis. Hydrogen atoms are adsorbed onto the surfaces of grains and concentrated long enough for two atoms to react ![]()
The exothermal nature of the reaction liberates enough energy to desorb the new 2 molecule back into the gas phase. This pathway is very efficient at low temperatures and is the only plausible means of producing 2. Since 2 drives much of the gas-phase chemistry, this is an extremely important phenomenon.
Grain surfaces are thought to be fairly ``sticky'' and very efficient at collecting and holding atoms which collide with them. Over time, this adsorption causes certain species to become depleted in the gas-phase.
Once species are adsorbed onto the grain surface, they are held in close proximity to many other atoms and molecules. Reactions occur forming 2 and other species through a complicated and only partially understood series of reactions.
Finally, processed materials consisting of complicated molecules are released back into the gas phase by a variety of methods both sudden and continuous. Observations of certain clouds shows evidence of higher-than-expected abundances of complex molecules. Clearly these molecules must have formed on grains and then were desorbed by a variety of processes. It is the last three aspects of interstellar dust that will be focussed on in this paper.
Recently, Turner (1990) observed two doubly-deuterated species in the Ori(KL) molecular cloud. Given that ![]() in the universe in general,
in the universe in general, ![]() 2 should be around
2 should be around ![]() ; essentially undetectable. However Turner measured
; essentially undetectable. However Turner measured ![]() CO/
CO/![]() CO at
CO at ![]() , far in excess of the abundance predicted. This is good evidence for grain chemistry as there is no plausible method of creating such concentrations in the gas phase. (Measurements of
, far in excess of the abundance predicted. This is good evidence for grain chemistry as there is no plausible method of creating such concentrations in the gas phase. (Measurements of ![]() were also made, but with less conclusive results.)
were also made, but with less conclusive results.)
There are the observations of dozens of large, complex molecules observed in interstellar space. Many of these molecules are undoubtedly more efficiently formed through surface reactions than in the gas-phase.
The spectral signatures of molecular ices (most notably CO, 2O, and ![]() ) are subtly different from their gaseous fellows (B&C 90). These spectra have been observed providing direct evidence for some of the simpler molecular processes in grain mantles. Though these discoveries falls considerably short of proving the existence of complex molecules in grain mantles, they gives such theories considerably more weight.
) are subtly different from their gaseous fellows (B&C 90). These spectra have been observed providing direct evidence for some of the simpler molecular processes in grain mantles. Though these discoveries falls considerably short of proving the existence of complex molecules in grain mantles, they gives such theories considerably more weight.
Several pieces of evidence seem to point to grain structure being amorphous. Large grains are believed to be formed from aggregations of smaller grains (Lang 88). In the low-energy realm of dust, it is unlikely that collisions release enough heat to melt and re-crystallize the material. Furthermore, the quantities of light-element impurities mixed into any sort of grain matrix is likely to disrupt ordered structures.
Since grains accrete mantles of icy lighter material over time, we should look at the nature of these materials. Water ice, a common mantle material, has three solid phases at low pressure. Above 190K, water crystallizes into the familiar hexagonal lattice. Colder than 190K, a cubic structure is seen, but below 135K, ice forms an amorphous structure (Leger et al 79). Since the temperature we expect to find in dense clouds rarely rises about 30K, amorphous mantles seem most obvious.
 |  |
Finally there are observations of what may well be interstellar dust on earth. Particles captured in the stratosphere possibly from comets (believed to be formed from relatively unprocessed dust complete with icy mantles) show a highly fractal, amorphous structure (Lang 88, McDonnell 88) (figure 1a). It should be noted, however, that recent particles observed in the Murchison meteorite show a highly regular structure suggesting crystalline surfaces. (Bernatowicz & Walker 97) (figure 1b).
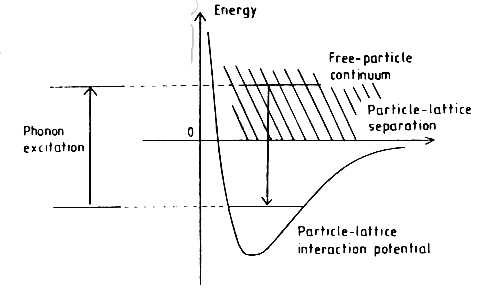
Figure 2: An energy diagram for the Leitch-Devlin and Williams (1985) model of adsorption.
A model by Buch and Zhang (1991) took advantage of numerical simulation to model hydrogen atoms impinging upon amorphous clusters of 115 water molecules. In this model, the adatoms were allowed to skip and roll around on the surface losing energy into phonon vibrations until they were captured. (Williams 93)
Both of these models predict values of S, the sticking efficiency, at close to 1.0. It is thought that ![]() is necessary to produce the observed quantities of 2 and some other molecules.
is necessary to produce the observed quantities of 2 and some other molecules.
The other side of this phenomenon is that, while atoms and molecules are being removed from the gas-phase, they are accreting and forming icy mantles on the grain surfaces. Grains that may form as ![]() m chunks of silicates or carbonaceous materials in the atmospheres of stars are now much larger due to their deep refractory mantles. Brown (1988) estimates that a 100 molecule thick mantle should form around grains in as short as
m chunks of silicates or carbonaceous materials in the atmospheres of stars are now much larger due to their deep refractory mantles. Brown (1988) estimates that a 100 molecule thick mantle should form around grains in as short as ![]() years.
years.
Two methods of mobility are likely. Assume that the atom is held in a potential well with characteristic oscillation frequency ![]() and that an energy barrier of height
and that an energy barrier of height ![]() exists to the next potential well. The time to transfer to the next site by thermal hopping will be
exists to the next potential well. The time to transfer to the next site by thermal hopping will be ![]()
Alternatively, the particle can quantum tunnel in a time ![]()
where ![]() is the width of the lowest energy band (Williams 93). For the low temperatures seen by dust grains, quantum tunneling is expected to be several orders of magnitude faster than thermal hopping (B&C 90). It should be noted that mobility within ice operates on same principles as mobility on the surface. Thus adatoms can travel through the entire volume of the mantle greatly increasing the chances of reaction.
is the width of the lowest energy band (Williams 93). For the low temperatures seen by dust grains, quantum tunneling is expected to be several orders of magnitude faster than thermal hopping (B&C 90). It should be noted that mobility within ice operates on same principles as mobility on the surface. Thus adatoms can travel through the entire volume of the mantle greatly increasing the chances of reaction.
Hydrogen atoms are light enough that they will scan the entire surface of the grain very quickly. Heavier atoms move slowly and are essentially immobile with respect to the fast hydrogen atoms. Thus we expect hydrogenation of O, C, and N, not to mention formation of 2 to be the most common reactions.
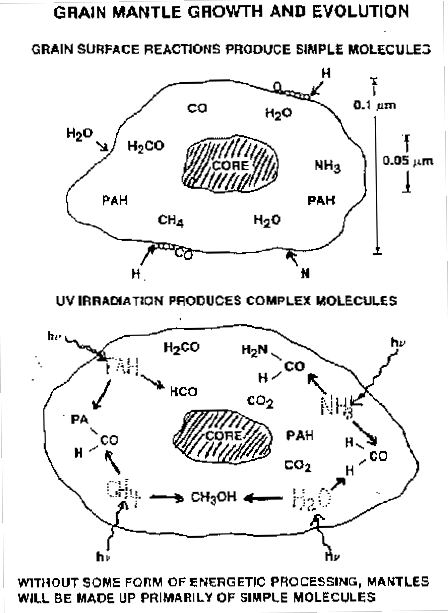
Figure 3: Schematics of some of the chemical processes taking place in grains (Allamandola et al 88). While simple molecules may form from hydrogenation and accretion (top), UV processing helps to form more complex species (bottom).
This leads us to another free parameter. Brown (1988) did some modeling of mantle composition over time as a function of the ratio of atomic hydrogen to molecular hydrogen and reached some interesting conclusions (figure 5). In clouds where there is sufficient atomic hydrogen, all surface atoms are ``immediately'' hydrogenated

These three compounds, plus CO accreted from the gas-phase will make up the mantle of the grain. In clouds with lower initial atomic hydrogen abundances (around 0.1%), the paucity of hydrogen limits the fast hydrogenation and allowed more complicated molecules to form.
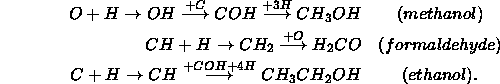
In clouds where all the available hydrogen has been converted to 2 , the mantle will be formed of elemental O, C, and N which will very slowly react with each other to form ![]() , CO and NO. This last case probably never happens in physical situations.
, CO and NO. This last case probably never happens in physical situations.
Other reactions can take place between peices of processed material. Incoming UV photons may break bonds in molecules and give enough energy to the products to catalyse other reactions (figure 3).
Micro-continuous processes account for some desorption (B&C 90). These are processes in which individual grains are continuously losing material. One such process is the familiar exothermic reaction ejection as seen in 2 formation; a product species near the surface is created with sufficient kinetic energy to break free from the surface. There are other permutations on this theme (Williams 93) such as neighbor ejection and chain reaction ejection. In the former, a product is created with sufficient energy to desorb both itself and a neighboring particle. In the latter, a reaction deep within the grain mantle releases energy into phonons and heat and cause particles closer to the surface to desorb. These processes depend critically on the grain structure and are not well understood.
Macro-continuous desorption may result in the destruction of particular grains, but is continuous throughout time on a larger scale. Sudden increases from high-energy photons can dump enough energy into a grain mantle that it explodes leaving a bare core. Likewise, a cosmic ray collision is likely to heat a cylindrical volume through the grain causing either a pair of hot-spots or a total explosion of mantle material. Cosmic rays can also induce UV photons in the neighborhood of a grain. These high energy photons will then strip the mantles off all nearby grains.
Finally, violent desorption is characterized by the simultaneous destruction of a large fraction of the grains in the cloud. Passing shocks can heat the local gas and bombard grains with high-speed particles. Periods of starformation can not only cause these shocks, but cause the local temperature to rise. Thus the grain mantles sublimate back into the gas phase. While star formation usually signals the end of the status quo for a particular cloud, shocks simply reset the chemical evolution of a cloud on a timescale of ![]() years.
years.
Here, however, lies the problem. There are too many free parameters to create any one all-encompassing model. Typical of the modelling efforts is one by Brown and Charnley (1990) based on the equations
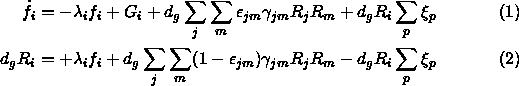
The details are not particularly relevant to this discussion except the first equation has to do with the gas abundance of species i while the second equation refers to the mantle abundance. The first term on the right in each equation deals with accretion. The second term (absent in equation 2) handles gas-phase production of species i. The third term handles the surface reaction production from parent species j and m and the fourth term handles the continuous desorption by process p. The accretion rate ![]() can be written out explicitly as
can be written out explicitly as
![]()
with a dependance on ![]() the sticking factor. Needless to say, there are quite a lot of unknowns in equations 1 and 2 (the desorption probabilities
the sticking factor. Needless to say, there are quite a lot of unknowns in equations 1 and 2 (the desorption probabilities ![]() just for starters) even if most of the physical conditions in equation 3 are known (mass, density, temperature, etc).
just for starters) even if most of the physical conditions in equation 3 are known (mass, density, temperature, etc).
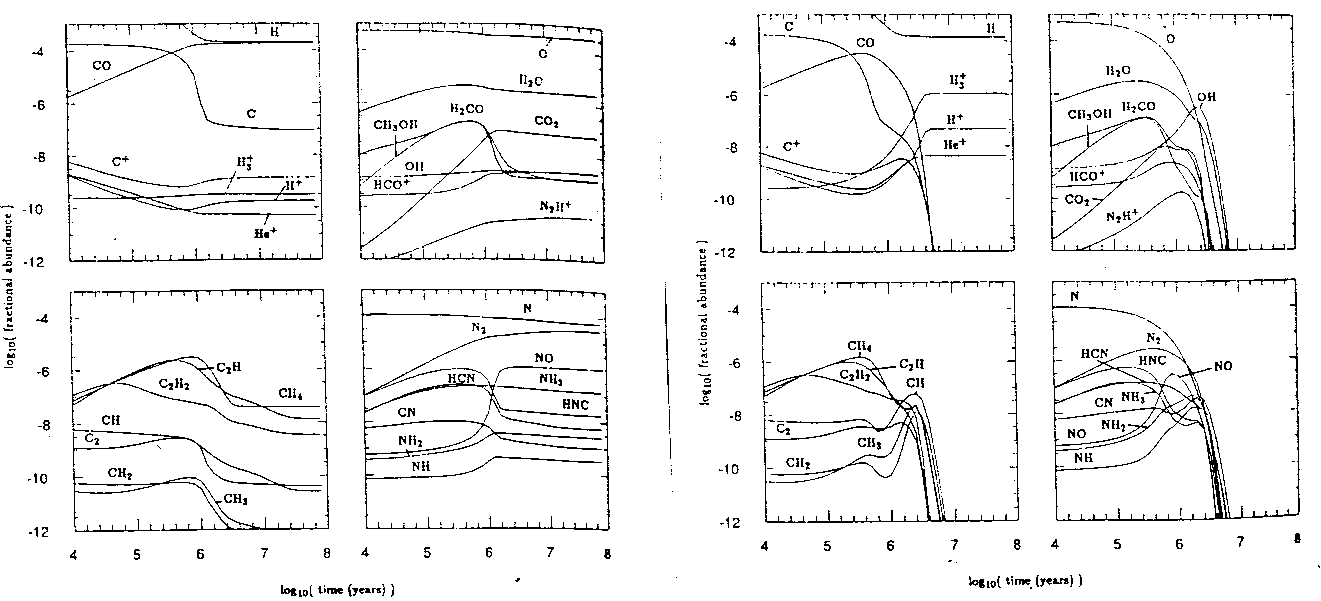
Figure: Two models illustrating the effects of the parameter S on molecular cloud gas-phase abundances. On the left we have S=0-the conventional model in which dust plays no chemical role. On the right S=1-perfect adsorption showing freeze-out after a few million years. (B&C 90)
Undaunted by this many-dimensional parameter space, Brown and Charnley set out and calculated some models (figure 4) for a number of different values of the stickiness. It can be seen that, while the two models are similar for small times, after a few hundred thousand years, the models show radically different evolution. While the model has certain limitations (grains are treated as crystals, only a limitted number of reactions are concidered) and certain parameters are not explored, we can see no convergence toward one final state.
Another example of this sensitivity to initial conditions can be seen in the work of Brown (1988) who varied the initial fraction of atomic to molecular hydrogen in the gas phase as described above. Two variations are shown in figure 5. Again, they show quite different evolutions from a small adjustment of only one parameter.
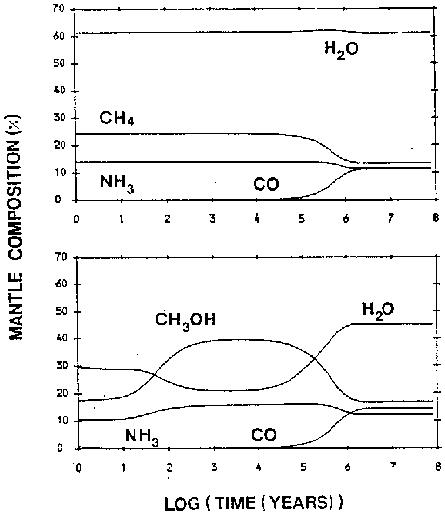
Figure 5: Two plots by Brown (1988) illustrating the mantle composition dependance on the initial H/2 ratio. The top plot shows an atomic hydrogen rich system with H/2=0.01 while the bottom plot shows H/2 =0.001.
A third example is given by Williams (1993) who modelled the chemistry in a static cloud given two different pathways to macro-continuous desorption (figure 6). Once again, dramatically different results are obtained given the relative efficiencies of two modes of desorption.
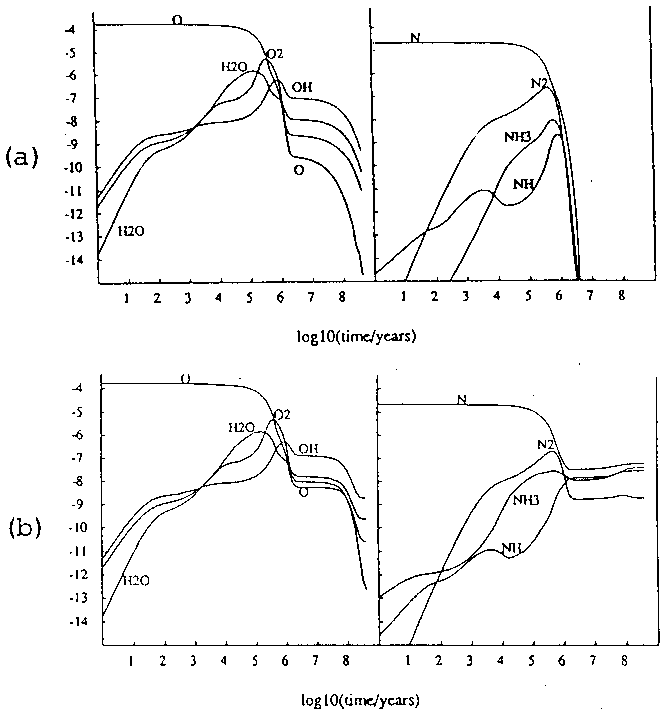 Figure 6: Models of gas-phase abundance in a dense cloud given (a) desorption by cosmic rays and (b) desorption by UV photons. (Williams 93)
Figure 6: Models of gas-phase abundance in a dense cloud given (a) desorption by cosmic rays and (b) desorption by UV photons. (Williams 93)
Furthermore, existing models feature too many free parameters. As mentioned above, three key parameters seem to be the surface characteristics (stickiness), initial atomic hydrogen fraction, and the efficiency of various desorption pathways. Add to this an imperfect knowledge of the chemical pathways involved and we can better grasp the magnitude of the task at hand.
While more observations and modelling must be done, we need a broader base of fundamental research upon which to rest it. Through the work of Pirronello (1996) and others in the laboratory creation of dust and measurements of sticking probabilities and reaction rates, we are getting a better fundamental understanding.
There is no substitute for actual field samples. The Stardust mission, scheduled for launch in 1999, is designed to collect samples of interstellar dust and dust from the coma of a comet. Upon return to earth in 2006, these samples will undoubtedly greatly increase our knowledge at the most fundamental level.
The command line arguments were:
latex2html dust2e.tex.
The translation was initiated by Charles Danforth on Mon Jan 26 22:05:24 EST 1998